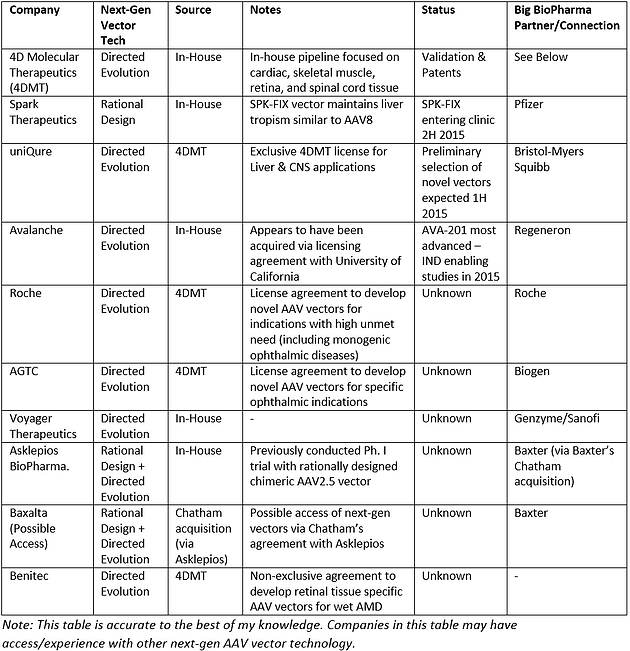
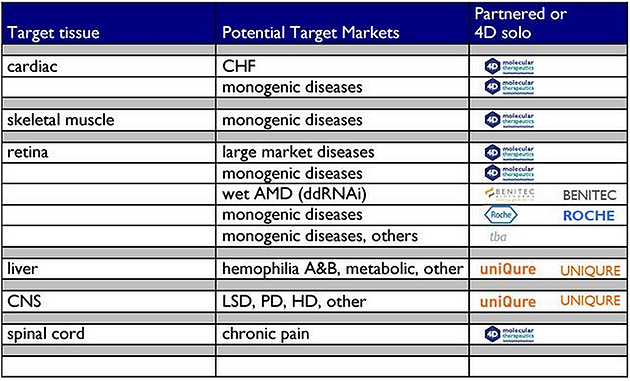
Category Archives: Benitec
Benitec – Carl Stubbings Interview on Sky Business News
Carl Stubbings Interview on Sky Business News
DSMB recommendation and HCV trial update of Benitec.
Sydney Australia, 21 July 2014: RNAi therapeutics company Benitec Biopharma
Limited (ASX: BLT, OTC: BTEBY) is pleased to provide an update regarding
recommendations from the Data Safety Monitoring Review Board (DSMB),
observations from the first patient dosed, and patient recruitment for the USJbased
Phase I/IIa TTJ034 clinical trial. TTJ034 is a ddRNAiJbased therapeutic, designed to
treat and potentially cure hepatitis C (HCV) with a single injection.
DSMB recommendation and observations from first patient dosed
Following review of data from the first patient treated with TTJ034 on 29 May 2014,
the … Read more
Calimmune stem cell therapy, HIV cure update.
Calimmune, Inc. today announced that encouraging results from a first group of participants indicates the company is ready to begin treating a second cohort in a clinical trial involving the use of Cal-1, an innovative gene-based stem cell therapy to help protect individuals infected with HIV from progressing to AIDS.
“While still early in clinical development, this announcement demonstrates real progress towards this mission. The accomplishment of Calimmune’s team is a great example of how CIRM partnerships are working to impact patient’s lives today.”
Calimmune was given approval to move … Read more
Benitec Director – Dr. Melvyn John Bridges.
Dr. Melvyn John Bridges B AppSc FAICD was Independent NonExecutive Director of Campbell Brothers Ltd since September 29 2009. He has over 30 years of experience in the biotechnology and healthcare industries. During this period he founded and managed diagnostics biotechnology and medical device businesses. He is currently Chairman of Alchemia Limited ImpediMed Limited and resigned from the post of NonExecutive Director of Benitec Limited . He also represent Chairmanship of Tissue Therapies Limited . He was previously Chairman of Peptech Limited Incitive Limited and a nonexecutive director of Genera … Read more
Alnylam and Benitec
Alnylam (ALNY) is a $5.1B company and Benitec Biopharma (BTEBY, BNIKF) is a $120M company. Both are biothech companies working in the field of RNAi (RiboNucleic Acid Interference). Both have access to a wide patent estate: Alnylam’s estate covering Small Interfering RNA (siRNA) and Benitec’s estate covering DNA Directed RNAi (ddRNAi and shRNA).
In theory they are competitors, each looking to silence genes which produce harmful levels of proteins. However, in reality this competition has not been manifest. Alnylam has concentrated its pipeline efforts primarily on rare … Read more
Benitec Start Dosing With TT-034 In Patients With Hepatitis C
Sydney Australia, 29 May 2014: RNAi based therapeutics company, Benitec Biopharma Limited (ASX: BLT, BNIKF in USA) is pleased to announce that it has dosed the first patient in its ‘first in man’, Phase I/IIa clinical trial for TT-034, a ddRNAi&based therapeutic, designed to treat and potentially cure hepatitis C (HCV) with a single injection.
Benitec Biopharma’s CEO and Managing Director, Peter French said, “The commencement of this clinical trial of TT-034 represents a landmark in the Company’s history. The trial is the first time Benitec’s gene silencing technology, ddRNAi, … Read more