Nitric oxide has become one of the most studied molecules in human physiology. The 1998 Nobel Prize-winning discovery triggered an eruption of research that now confirms nitric oxide’s natural ability to prevent clotting, regulate inflammation, revitalize tissue, kill invading microorganisms, and even eradicate cancer cells.
Novan Therapeutics… Read more
Monthly Archives: March 2015
Cystic fibrosis Cure with RNAi Technology – ProQR
What is cystic fibrosis?
Cystic fibrosis (CF) is a genetic disease affecting more than 70,000 patients worldwide. CF patients have viscous mucus accumulating in their vital organs. The mucus clogs tubes and organs thereby disrupting several processes in the body.
Pancreatic enzymes, necessary to digest food, are blocked from entering the intestines, so CF patients are not able to digest food by themselves. The thick layer of mucus in the lungs is a great environment for destructive bacteria. The thick mucus makes it hard to clear the lungs from these … Read more
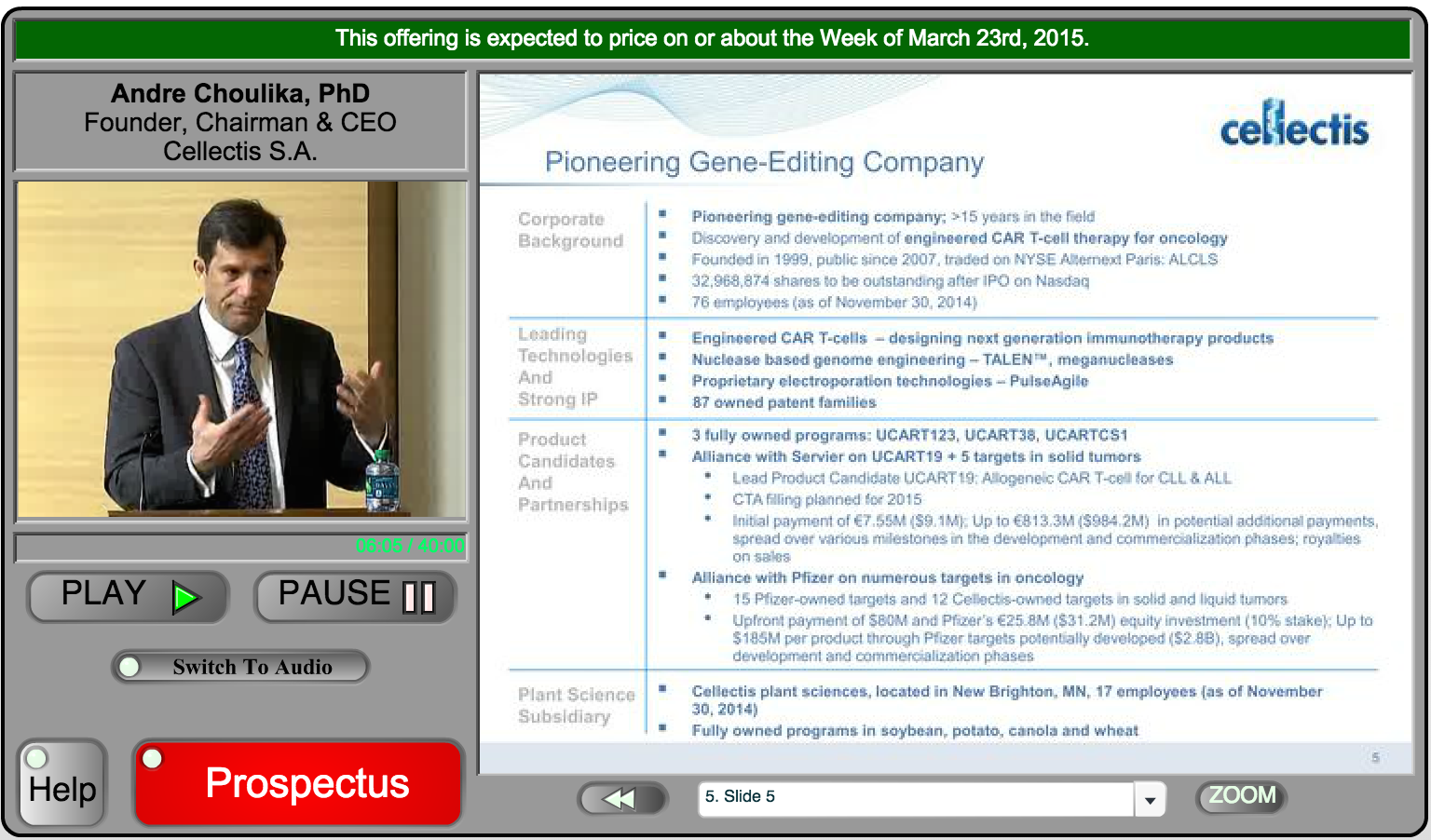
Cellectis – CAR T-Cell Therapy
All you need to know about Cellectis :
4 branchs :
-Oncology
-Sceil
-Cellectis Bioresearch
-Cellectis Plants Science (CPS)
Corporate presentation : urlz.fr/1AWL
http://www.capitalpress.com/Nation_World/Nation/20141125/new-gmo-potato-avoids-usda-regulation
http://sceil.com/
http://www.ascrnetwork.com/index.php?option=com_content&view=article&id=3014:cellectis-bioresearch-inc-wins-a-69-million-contract-with-the-national-institutes-of-health-on-ips-cells&catid=102
http://infocampo.com.ar/nota/campo/67136/cellectis-plant-sciences-una-emergente-que-puede-revolucionar-el-mejoramiento-vegetal
http://www.cellectis.com/en/content/cellectis-step-towards-new-therapy-aids-cellectis-publishes-genome-engineering-advance
http://www.nature.com/srep/2015/150130/srep08150/full/srep08150.html… Read more
CRISPR vs TALEN vs ZFN vs RNAi vs Homing Meganucleases
-Homing meganucleases are enormous and old-school
-ZFNs are compact (28:1 for input DNA:target) but obviously hard to target, so they may have specialized applications
-TALENs can be synthesized solid-phase, but they’re pretty huge (I think it was either 100 or 1k:1). The modularity is an improvement over ZFNs, which is why they were in fashion
-CRISPR are of course the best (1:1), although the extra machinery may be tough to deliver. Also, I’m curious as to the packing of the RNA–I feel like that might pose a problem for delivery? … Read more
CRISPR/Cas9 and Targeted Genome Editing
The development of efficient and reliable ways to make precise, targeted changes to the genome of living cells is a long-standing goal for biomedical researchers. Recently, a new tool based on a bacterial CRISPR-associated protein-9 nuclease (Cas9) from Streptococcus pyogenes has generated considerable excitement (1). This follows several attempts over the years to manipulate gene function, including homologous recombination (2) and RNA interference (RNAi) (3). RNAi, in particular, became a laboratory staple enabling inexpensive and high-throughput interrogation of gene function (4, 5), but it is hampered by providing only temporary … Read more
CRISPR/Cas9 System for Targeted Genome Modification
The CRISPR/Cas9 system, Origene’s latest tool in genome editing, is a complete system for highly specific gene knockout with low cell toxicity. The CRISPR/Cas9 technology uses co-expression of a Cas9 protein with a guide RNA vector expressed from the human U6 polymerase III promoter. Cas9 unwinds the DNA duplex and cleaves both strands upon recognition of a target sequence by the guide RNA.
CRISPR/Cas9 Kit Features:
Complete kit for gene knockout using CRISPR
Two guide RNA vectors in for efficient cleavage
Knockin GFP-Puro for selection
pCas-Guide-scramble included as a negative … Read more
Gene Therapy Studies for the Treatment of HIV Infection
CureVac – Messenger RNA (mRNA) Technology
CureVac is pioneering the use of natural and chemically unmodified mRNA as a data carrier to instruct the human body to produce its own proteins capable of fighting a wide range of diseases. CureVac’s novel technology is the broadest and most advanced mRNA therapeutic platform and allows for rapid, low-cost production of multiple drugs and vaccines. Additionally, CureVac’s mRNA vaccines are thermostable, which eliminates the demand for cold-chain storage and infrastructure, a major challenge in the vaccine supply of most developing countries.
CureVac’s RNA Technology – Inspired by Nature
CureVac’s
non-small cell lung cancer got FDA approval
 Bristol-Myers Squibb ($BMY) picked up FDA approval to use its new oncology treatment in lung cancer, significantly boosting the value of a therapy already expected to bring in billions each year.
Bristol-Myers Squibb ($BMY) picked up FDA approval to use its new oncology treatment in lung cancer, significantly boosting the value of a therapy already expected to bring in billions each year.
The FDA’s latest approval of Opdivo, already cleared for melanoma, comes just days after the agency accepted Bristol-Myers’ application and more than three months ahead of schedule. Bristol-Myers’ treatment is designed to galvanize an immune system attack on tumors by blocking a pathway called PD-1, which, left unchecked, allows cancer cells to pass undetected by the body’s natural defenses.… Read more
Novartis Sold its RNAi Technology to Arrowhead
Arrowhead acquires patents as well as intellectual property rights to Alnylam’s RNAi tech, 30 gene targets Novartis has picked from its partnership and a pipeline with three candidates that had generated preclinical data. And it follows their 2011 deal to buy out Roche’s spurned RNAi work, providing some added boasting rights to being a leader in the field.
“This is an important deal for us. Novartis has been working in the RNAi field for over a decade and their developments in proprietary oligonucleotide formatting and modifications are some of the … Read more