Sydney Australia, 29 May 2014: RNAi based therapeutics company, Benitec Biopharma Limited (ASX: BLT, BNIKF in USA) is pleased to announce that it has dosed the first patient in its ‘first in man’, Phase I/IIa clinical trial for TT-034, a ddRNAi&based therapeutic, designed to treat and potentially cure hepatitis C (HCV) with a single injection.
Benitec Biopharma’s CEO and Managing Director, Peter French said, “The commencement of this clinical trial of TT-034 represents a landmark in the Company’s history. The trial is the first time Benitec’s gene silencing technology, ddRNAi, has been used systemically in
patients. The primary objective of this first trial is to demonstrate that TT-034 can be used safely in patients with HCV. Preclinical work in non-human primates demonstrated very low toxicity
results at therapeutically relevant doses, and we’re hopeful that we will see the same favourable tolerability in humans. In addition, we will be able to assess the impact of TT- 034 treatment on HCV viral load in these patients, and this important efficacy marker
constitutes one of the secondary endpoints of this study.”
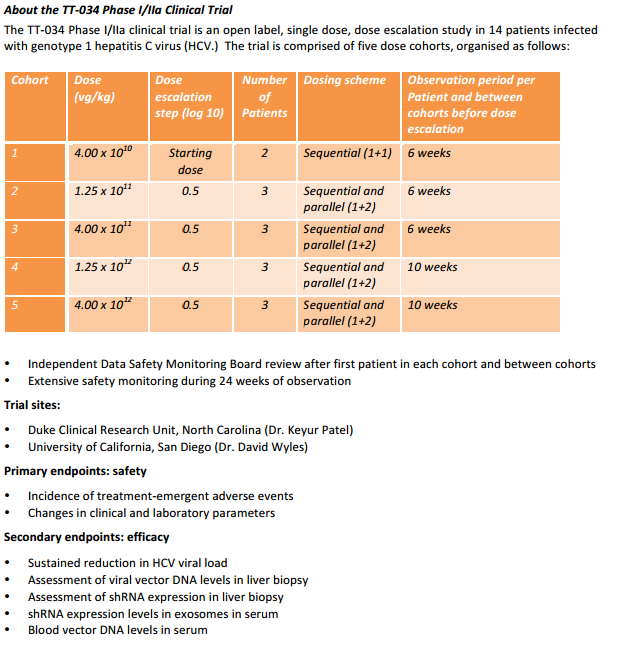
The TT&034 Phase I/IIa clinical trial is an open label, dose escalation study in a total of 14 patients chronically infected with HCV genotype 1. Initial patient cohorts will be treated
with a sub&therapeutic dose of TT-034 to ensure that there are no unexpected safety concerns, before proceeding to higher, potentially therapeutic doses. An expert medical panel, the Data Safety Monitoring Board (DSMB), which is independent
of Benitec, will carefully assess the data from each patient, in particular the safety data. The DSMB assessment will occur after the first patient in each cohort and between
cohorts, and will determine the timing of each subsequent dosing.